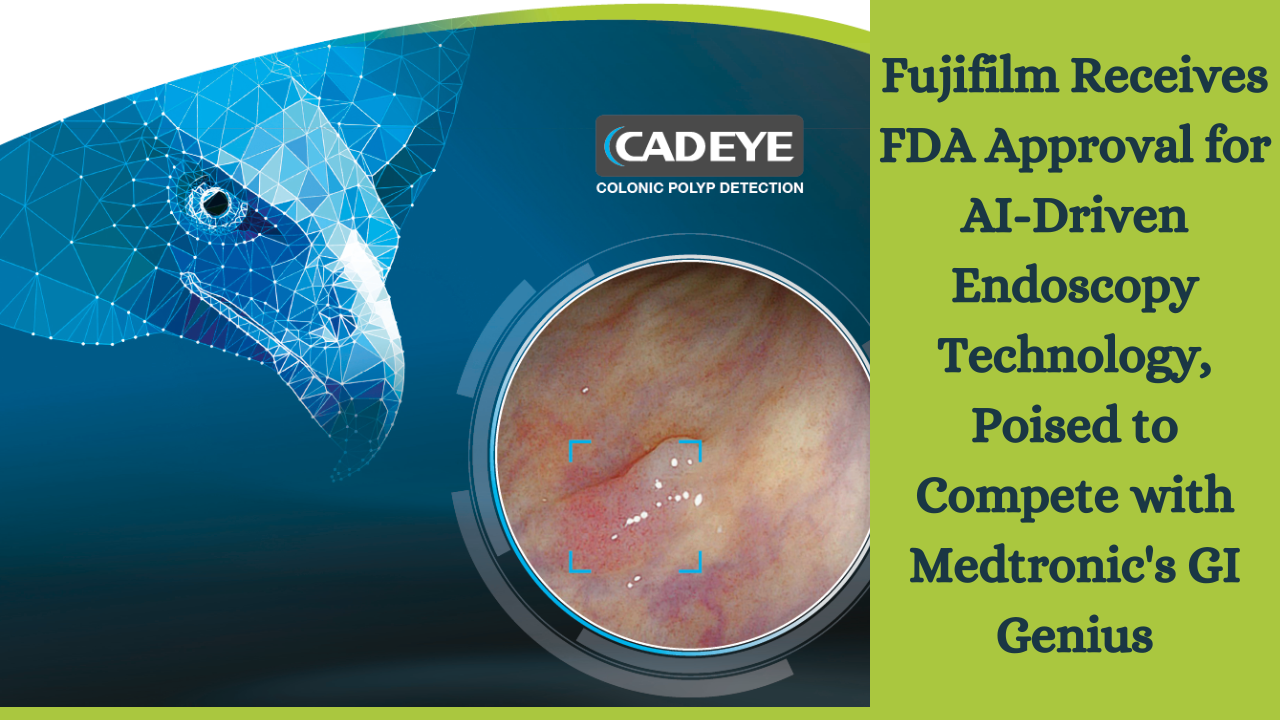
Fujifilm announced today that the FDA has granted 510(k) clearance for its CAD Eye AI-driven system for endoscopic imaging. CAD Eye assists during colonoscopy procedures in real-time detection of large bowel mucosal wounds like polyps and adenomas. It aids in identifying and removing pre-cancerous wounds before they develop into cancer, based on their size, shape, and color, with the help of endoscopists. This system enters a market currently dominated by Medtronic and its GI Genius system.
GI Genius, the first AI-driven system for polyp detection during colonoscopy, became available in the United States in 2021. For more on Medtronic’s AI efforts with GI Genius, read here.
Both tools were designed to assist endoscopists in detecting colon mucosal wounds. CAD Eye works alongside white-light imaging and linked-color imaging (LCI). Additionally, a better visualization mode is included in the Fujifilm system’s red spectrum, enhancing mucosal visualization. According to Anthony Borrelli, Executive Director of Endoscopic Products and Marketing at Fujifilm Healthcare Americas Corporation, the capabilities of LCI, in addition to HD white light, make a significant difference in competitive GI Genius.

Fujifilm’s system includes a coordinated expansion unit (Fujifilm EX-1) and endoscopic support software (EW10-EC02). AI image processing is integrated with the system’s processor and endoscope through CAD Eye, based on the Eluxeo Endoscopic Imaging System of the CAD Eye company.
The company’s plan is to make CAD Eye commercially available in the spring season after completing limited diagnostic testing in the market.
According to GM of Fujifilm Healthcare America’s Endoscopy Division, Taizo Fujita, “Every day, we take pride in providing what we believe is the highest quality imaging and optics, equipping endoscopists with these tools to tackle the public health challenge.”
“Today, we are particularly excited to advance with the introduction of CAD Eye, which dramatically enhances the quality of colonoscopy, further empowering clinicians,” he added enthusiastically.
Moreover, Borrelli stated that Fujifilm’s CAID AI system’s internal development and AI training distinguish it from the competition. Developers trained the system on images taken with Fujifilm endoscopic imaging equipment.
“Fujifilm believes it is a significant force because the company is known for its best optics and image quality – especially in its endoscopic imaging system – which we believe strengthens the CAD Eye product,” he said.
Furthermore, it offers smooth integration into the doctor’s workflow. Doctors can activate and deactivate CAD Eye on the endoscope handle, orient the tool toward the lesion, or seek help without having to remove their eyes from the patient.
“It is evident from customer feedback that it is highly effective in their workflow and making a significant difference,” Borrelli continued.
Regarding Fujifilm’s CAD Eye system, further,
the company developed CAD Eye using deep learning technology at the Global AI Technology Center in Tokyo. Fujifilm validated the system using histologically confirmed polyps in medical images.
A news discharge expresses that the innovation finds wounds that may be difficult to recognize, similar to level injuries, wounds that are outside the field of perspective on the endoscope, and a few injuries in a solitary casing. When CAD Eye identifies a suspicious polyp, it automatically generates both visual and auditory alerts. Visual alerts overlay, but do not interfere with current clinical images in the existing workflow.
Fujifilm said studies have shown significant progress in detecting and diagnosing large bowel cancer with CAD Eye, without the need for AI during conventional high-definition colonoscopy. Additionally, it operates without any added time during procedures.
Furthermore, compared to high-definition conventional colonoscopy, Fujifilm observed 17% more adenomas with Fujifilm’s Fujifilm CAD Eye system. The organization said it recognizes colorectal neoplastic injuries at a level that can measure up to a specialist and is prevalent in beginning finding.
“I am pleased with FDA’s approval of Fujifilm’s AI CAD polyp detection algorithm, which we believe advances our ability to not only identify wounds ahead of time that could lead to colorectal cancer but also visibly reduces the risk of missed wounds, improves our accuracy, and results in better patient outcomes,” said Dr. Surani Parsa of Swedish Medical Center. “This denotes a significant development in our capacity to safeguard patient wellbeing by embracing mechanical advancements in gastric medical services.”